| Product | Discovery | Pre-clinical Studies | Phase I Studies | Phase II Studies | Phase III Studies | BLA / MAA | Market |
|---|---|---|---|---|---|---|---|
| TMB-365 and TMB-380 combination | Discovery
|
Pre-clinical Studies
|
Phase I Studies
|
Phase II Studies
|
Phase III Studies
|
BLA / MAA
|
Market
|
TMB-365/TMB-380 (TMB-365 and TMB-380 combination)
TMB-365/TMB-380 Injection
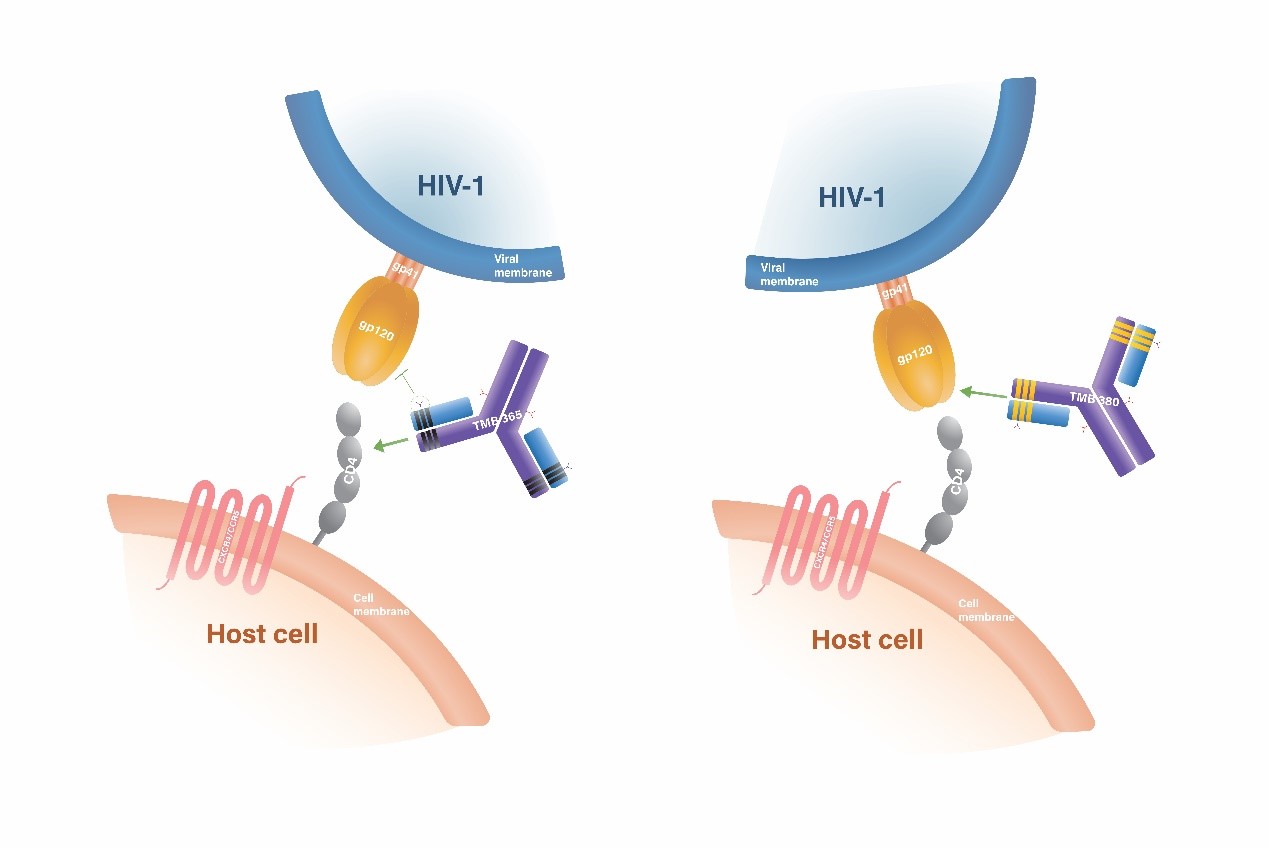
TMB-365/TMB-380 is a clinical stage long-acting monoclonal antibody combination therapy for HIV . It is positioned as a complete regiment for HIV maintenance therapy. The designated dual antibody combination consists of two long-acting monoclonal antibodies with distinct and highly differentiated modes of action against HIV infections. TMB-380 (also known as VRCO7-523LS) is a highly potent, long-acting, broadly-neutralizing antibody that targets the CD4 receptor binding sites (CD4bs) on the HIV-1 envelope glycoproteins. On the other hand, TMB-365 is a highly potent, long-acting, broadly-blocking antibody that attaches to the cell surface CD4 receptors, preventing HIV from entering CD4+ cells. In Phase 1b/2a clinical study, TMB-365/TMB-380 is proven to be well-tolerated, safe, and long-acting when administrated intravenously. It is currently in Phase 2 clinical study to evaluate its efficacy when administered every two months.
Clinical trial for the combination of TMB-365 and TMB-380
Based on the available data, both TMB-365 and TMB-380 have superior antiviral activities with no safety concerns. The pharmacokinetic profiles of both antibodies indicate the potential of bi-monthly or quarterly dosing. By combining these two antibodies, the combination of TMB-380 and TMB-365 takes advantage of complementary mechanisms for inhibiting HIV entry. TaiMed has submitted an IND application to US FDA for the combination of TMB-365 and TMB-380.
A phase 1b/2a clinical trial with adaptive design was proposed for viral-suppressed HIV-1 patients. The study will investigate the safety and efficacy of the combination at different dose levels every 8 or 12 weeks.
-
2019-10-012022
2022: Initiation of a phase-1b/2a clinical trial for the combination of TMB-365 and TMB-380


