| 產品 | 藥物探索 | 臨床前試驗 | 臨床一期 | 臨床二期 | 臨床三期 | 藥證申請 | 上市銷售 |
|---|---|---|---|---|---|---|---|
| TMB-365 and TMB-380 雙抗體複方組合 | 藥物探索
|
臨床前試驗
|
臨床一期
|
臨床二期
|
臨床三期
|
藥證申請
|
上市銷售
|
TMB-365/TMB-380 雙抗體複方組合
TMB-365/TMB-380 注射液
TMB-365/TMB-380 是用於HIV治療,目前在臨床研發階段的長效型雙單株抗體複方雞尾酒療法,。它被定位為HIV維持治療的完整方案。該抗體複方雞尾酒藥物包括兩種長效型單株抗體,在對抗HIV感染上,這兩種單株抗體各自具有不同且高度差異化的作用機制。TMB-380(又名VRCO7-523LS)是一種高效、長效、廣效型對病毒具中和性的單株抗體,其標靶是HIV-1病毒鞘蛋白上的CD4受體結合位點(CD4bs)。而另一方面,TMB-365也是一種高效、長效、廣效型對病毒入侵具阻絕性的單株抗體,它能附著在細胞表面的CD4受體上,從而防堵阻礙HIV侵入CD4+免疫細胞。 在該抗體複方藥物1b/2a期臨床研究中,靜脈注射TMB-365/TMB-380被證實具有良好的耐受性、安全性和長效性。目前它正在進行第2期臨床研究,以評估每兩個月施用一次的療效。
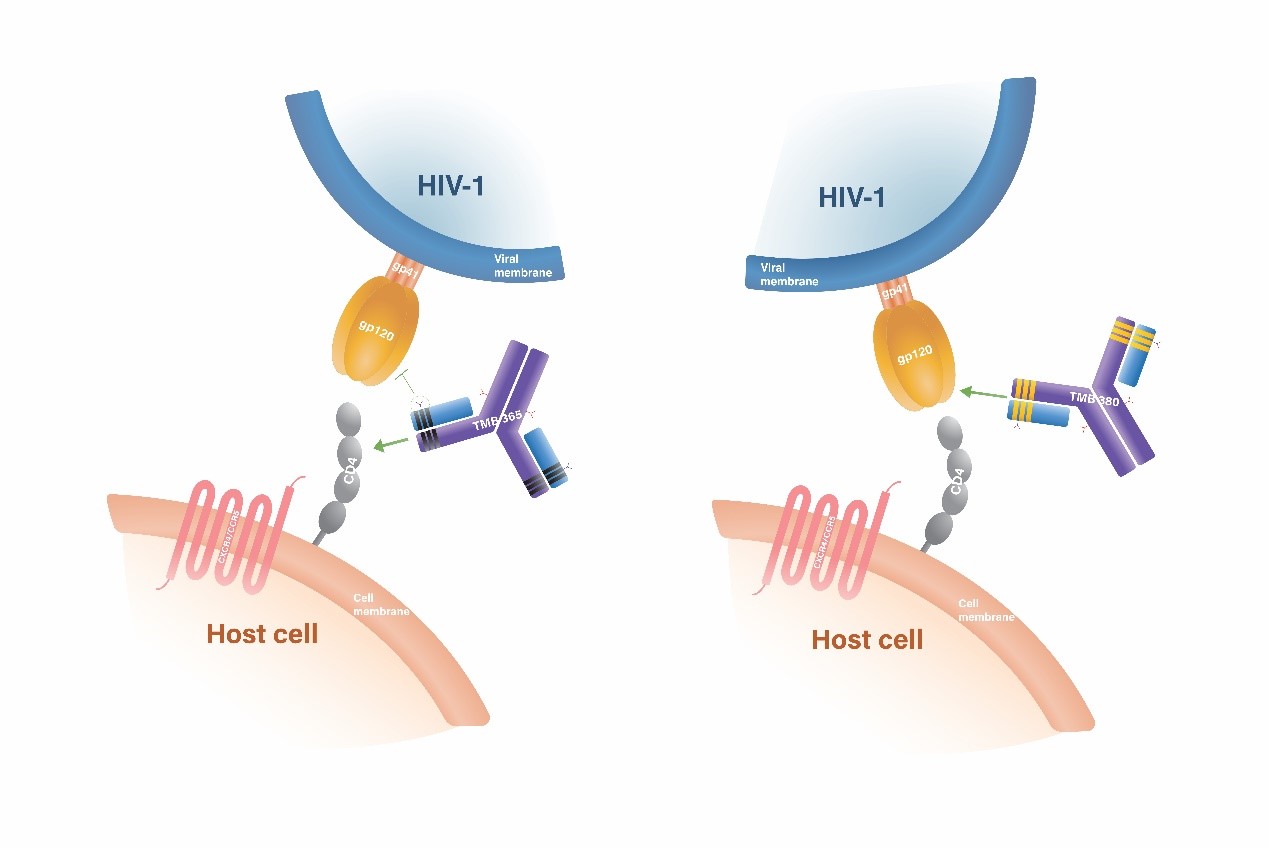
TMB-365/TMB380合併使用臨床實驗
根據現有數據分析,TMB-365與TMB-380都具有卓越的抗愛滋病毒能力,而且藥物動力學表現都顯示出每兩個月或每三個月給藥一次的可能性,藉由結合TMB-365與TMB-380不同的作用機制,中裕發展出以TMB-365及TMB-380合併使用的臨床1b/2a試驗設計,並於2022年向美國FDA申請IND。
利用調整性試驗設計,針對使用抗病毒藥物組合治療且體內病毒量受到抑制的HIV-1感染者,評估在不同劑量下、每8週或每12週靜脈注射的TMB-365和TMB-380合併療法的安全性與有效性。
里程碑
-
2019-10-012022
啟動TMB-365/TMB-380合併使用的臨床1b/2a試驗


